Diabetes—is a condition in which a person has a high blood sugar (glucose) level as a result of the body either not producing enough insulin, or because body cells do not properly respond to the insulin that is produced. Insulin is a hormone produced in the pancreas which enables body cells to absorb glucose, to turn into energy. If the body cells do not absorb the glucose, the glucose accumulates in the blood (hyperglycemia), leading to various potential medical complications, and eventually death.
In Type 1 Diabetes the patient's body produces no insulin, which must be externally supplied by injection. In Type 2 Diabetes the patient may not produce enough insulin, or may be Insulin-resistant. In both cases, proper Insulin levels must be maintained by monitoring the patient's blood glucose level by blood sampling. This is usually done by obtaining a blood sample manually and testing with an enzyme for glucose level.
Prevalence of Diagnosed and Undiagnosed Diabetes among People Ages 20 Years or Older, United States, 2007: 23.5 million, or 10.7 percent, of all people in this age group have diabetes. Age 60 years or older: 12.2 million, or 23.1 percent, of all people in this age group have diabetes. Huge problem, and huge market.
A glucose meter (or glucometer) is a medical device for determining the approximate concentration of glucose in the blood. It is a key element of home blood glucose monitoring (HBGM) by people with diabetes mellitus or hypoglycemia. A small drop of blood, obtained by pricking the skin with a lancet, is placed on a disposable test strip that the meter reads and uses to calculate the blood glucose level. The meter then displays the level in mg/dl or mmol/l.
Since approximately 1980, a primary goal of the management of type 1 diabetes and type 2 diabetes mellitus has been achieving closer-to-normal levels of glucose in the blood for as much of the time as possible, guided by HBGM several times a day. The benefits include a reduction in the occurrence rate and severity of long-term complications from hyperglycemia as well as a reduction in the short-term, potentially life-threatening complications of hypoglycemia. Home monitoring is currently done by units such as this.
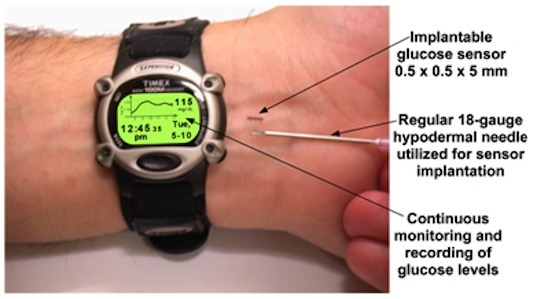
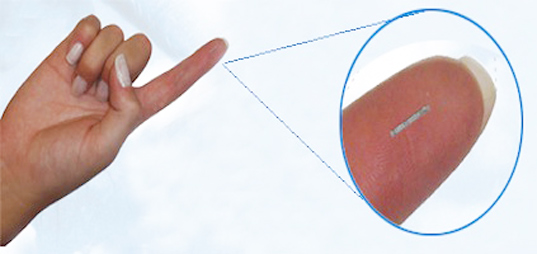
A less invasive method of measurement has long been sought. Recently an injectable sensor has been announced; while not yet an available product, we will use this sensor as the basis for our design project.

The Data Collection Device: Chronos
The Chronos is a Texas Instruments MPS430 very low power microprocessor-based device in the form factor of a watch. (This was my 2nd choice for a platform for this year's course, behind the Mini2440). Chronos can display a number and other data, but has no graphic capabilities.
For our purposes, we'll assume that Chronos has a light source on the underside which can be flashed to charge the Glucowizzard sensor.
The Sensor: Glucowizzard, article
For our purposes, you may assume that the Glucowizzard sensor has a compatible radio to the Chronos, operating at 915MHz. The sensor transmits an unsigned 16 bit number, which must be converted to Mg/DL (milligrams per deciliter) via a lookup table or other algorithm.
A previous product of similar purpose
Your design document covering these topics should be turned in by 11:30 p.m., Thursday, March 18, 2010, here.